One of the most common questions pre-med students ask is if they’ll have access to this essential chemistry reference during the MCAT (Medical College Admission Test) exam.
The short answer is, yes, you do get a periodic table on the MCAT, but it’s not the detailed version you might be used to.
Knowing what’s included and missing will help you prepare effectively. Here you will get to know how the MCAT periodic table is important and what you need to keep in mind for test day.
Do You Get a Periodic Table on the MCAT?
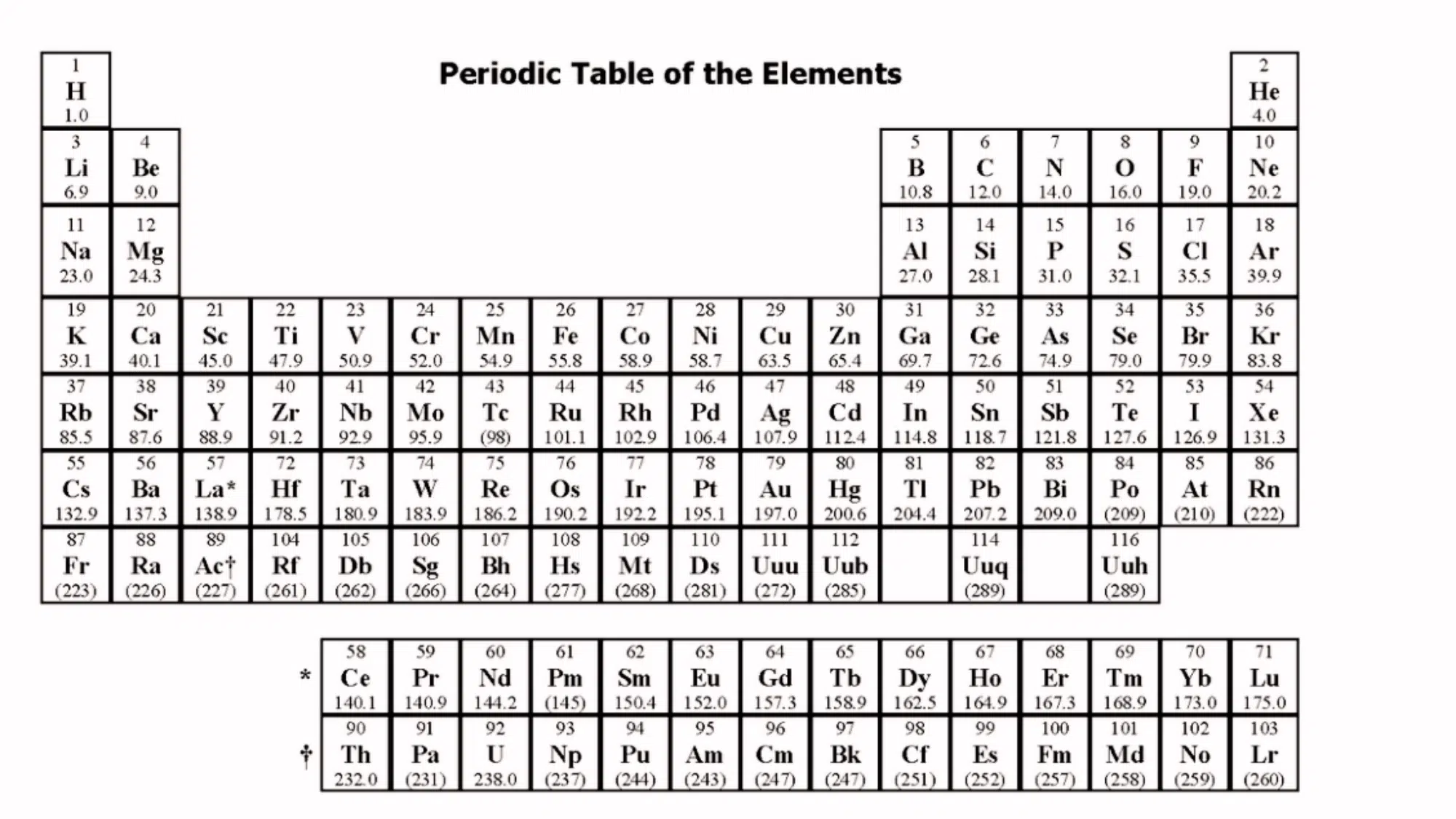
The answer to this question is yes, as per the official AAMC policy, a basic periodic table is provided as a reference during the MCAT.
It includes important element symbols, atomic numbers, and atomic weights, but lacks detailed chemical data.
The table is accessible digitally within the exam interface, which differs from traditional printed versions. Familiarizing yourself with its format will save time during the test.
Why is the MCAT Periodic Table so Important?
The table offers essential element symbols and atomic numbers, helping you quickly identify key elements. Atomic weights are also listed, but rounded and simplified.
Some information you may expect, like electron configurations or electronegativities, is missing, which you must memorize instead. The layout is more condensed and less detailed than standard classroom tables.
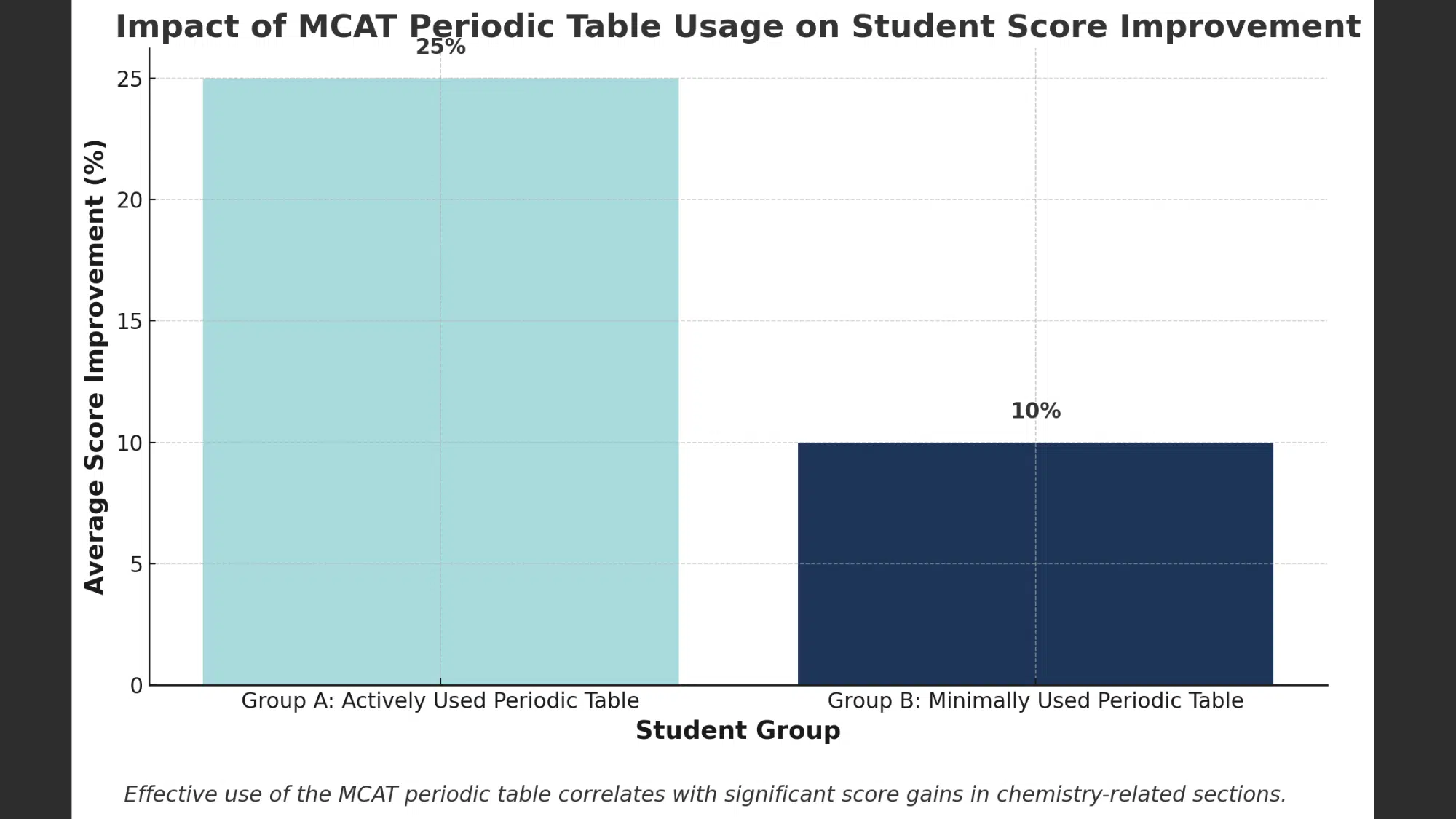
Over time, it has been observed that students who used the MCAT Periodic table have scored much better than those who didn’t.
What’s NOT Included that You Should Know
Important chemical properties are excluded from the MCAT table, such as electron configurations, ionization energies, and electronegativity values.
Common oxidation states are also omitted. Since these details are not provided, it’s crucial to commit them to memory before your exam to avoid surprises.
How to Access the Periodic Table During Your Exam

The periodic table is available through the computer interface, accessible via a toolbar or a clickable icon.
You can use it anytime during relevant questions, but not during breaks or transitions.
Practicing navigation and timing your use ahead of the exam day will help you avoid delays and manage your test time efficiently.
Key Elements and Properties to Memorize
Here are some Key elements and Properties you should memorize beforehand to ensure time saving in the exam hall.
- Memorize the first 20 elements, their symbols, and common ions and charges, as these are frequently tested.
- Understanding periodic trends, like atomic radius and electronegativity across groups and periods, is vital.
- Knowing group and period characteristics will enable faster problem-solving without over-reliance on the table.
MCAT Chemistry Topics Requiring the Periodic Table
General Chemistry Applications
The periodic table provides essential information like atomic numbers and atomic masses needed for balancing chemical equations and calculating molar masses, core skills tested in general chemistry on the MCAT.
General Chemistry Applications usually contain these important subparts:
- Atomic Numbers and Atomic Masses
- Electron Configuration and Valence Electrons
- Periodic Trends (Atomic Radius, Electronegativity, Ionization Energy, Electron Affinity)
- Stoichiometry and Molar Mass Calculations
- Chemical Bonding and Molecular Structure
- Balancing Chemical Equations
- Redox Reactions and Reaction Mechanisms
Organic Chemistry Connections
Familiarity with element valency, especially carbon, hydrogen, oxygen, and nitrogen, helps in understanding functional groups and bonding patterns critical to organic chemistry questions.
Organic Chemistry Connections usually contain these important subparts:
- Element Valency and Bonding Patterns
- Functional Groups and Structure
- Orbital Hybridization
- Electronegativity and Bond Polarity
- Reaction Mechanisms (Nucleophiles, Electrophiles)
- Molecular Geometry and Stereochemistry
Biochemistry Relationships
Quick recall of elemental properties supports understanding biochemical molecules and reactions, such as enzyme functions and metabolic pathways that depend on atomic behavior.
Biochemistry Relationships usually contain these important subparts:
- Enzyme Cofactors and Metal Ions
- Biochemical Molecule Composition and Elemental Roles
- Acid-Base Chemistry in Biological Systems
- Redox Reactions and Electron Transport Chain
- Metabolic Pathways and Element-Dependent Reactions
Physics Calculations Involving Elements
Some physics questions require knowledge of elemental constants and atomic structure, using the periodic table for solving problems involving energy levels and particle interactions.
Physics Calculations Involving Elements usually contain these important subparts:
- Atomic Mass and Nuclear Properties
- Isotopes and Radioactivity (Decay, Half-life)
- Spectroscopy and Atomic Structure
- Quantum Mechanics Related to Electron Behavior
- Nuclear Imaging and Radiation Physics
How to Study for MCAT Chemistry?
You need to study strategically if you wish to be among the top scorers of the MCAT. To do so, keep these things in mind.
- Practice chemistry problems both with and without the periodic table to strengthen recall.
- Use memory aids such as mnemonics or flashcards for key element facts.
- Timing yourself on practice tests will teach you when to refer to the table effectively and when to rely on memorized data.
- Integrate chemistry review with other MCAT sections for well-rounded preparation.
Questions to Practice for MCAT
For your MCAT preparation, it is essential to have a clear understanding of the variety of topics that span the exam’s four sections, including biological sciences, chemistry, physics, psychology, and reasoning skills.
To aid your study, a comprehensive PDF containing these questions with explanations is available.
Click here: MCAT Chemistry Practice
Common Mistakes Students Usually Make
These are some common mistakes that hamper students’ preparation:
- Relying too heavily on the provided table can slow down your pace.
- Not memorizing essential properties leaves gaps that affect answer accuracy.
- Poor time management, such as spending too long accessing the table, can eat into overall test time.
- Misunderstanding which details are included may confuse questions.
Wrap It Up!
The MCAT periodic table is a helpful but limited tool. Being familiar with what it offers, and what it omits, along with committing key information to memory, will give you an edge on test day.
Keep practicing efficient access and balance your use of the table with tested knowledge.
With solid preparation, you will approach the exam confidently and be ready to tackle the chemistry sections successfully.
All the best for your Exams!